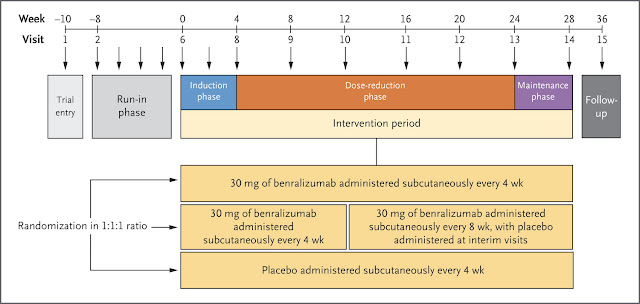
Many patients with severe asthma rely on oral
glucocorticoids to manage their disease. We investigated whether
benralizumab, a monoclonal antibody directed against the alpha subunit
of the interleukin-5 receptor that significantly reduces the incidence
of asthma exacerbations, was also effective as an oral
glucocorticoid–sparing therapy in patients relying on oral
glucocorticoids to manage severe asthma associated with eosinophilia.
In
a 28-week randomized, controlled trial, we assessed the effects of
benralizumab (at a dose of 30 mg administered subcutaneously either
every 4 weeks or every 8 weeks [with the first three doses administered
every 4 weeks]) versus placebo on the reduction in the oral
glucocorticoid dose while asthma control was maintained in adult
patients with severe asthma. The primary end point was the percentage
change in the oral glucocorticoid dose from baseline to week 28. Annual
asthma exacerbation rates, lung function, symptoms, and safety were
assessed.
Results
Of
369 patients enrolled, 220 underwent randomization and started
receiving benralizumab or placebo. The two benralizumab dosing regimens
significantly reduced the median final oral glucocorticoid doses from
baseline by 75%, as compared with a reduction of 25% in the oral
glucocorticoid doses in the placebo group (P<0.001 for both
comparisons). The odds of a reduction in the oral glucocorticoid dose
were more than 4 times as high with benralizumab as with placebo. Among
the secondary outcomes, benralizumab administered every 4 weeks resulted
in an annual exacerbation rate that was 55% lower than the rate with
placebo (marginal rate, 0.83 vs. 1.83, P=0.003), and benralizumab
administered every 8 weeks resulted in an annual exacerbation rate that
was 70% lower than the rate with placebo (marginal rate, 0.54 vs. 1.83,
P<0.001). At 28 weeks, there was no significant effect of either
benralizumab regimen on the forced expiratory volume in 1 second (FEV1),
as compared with placebo. The effects on various measures of asthma
symptoms were mixed, with some showing significant changes in favor of
benralizumab and others not showing significant changes. Frequencies of
adverse events were similar between each benralizumab group and the
placebo group.
Conclusions
Benralizumab
showed significant, clinically relevant benefits, as compared with
placebo, on oral glucocorticoid use and exacerbation rates. These
effects occurred without a sustained effect on the FEV1.
Free full text:
http://www.nejm.org/doi/full/10.1056/NEJMoa1703501#Top