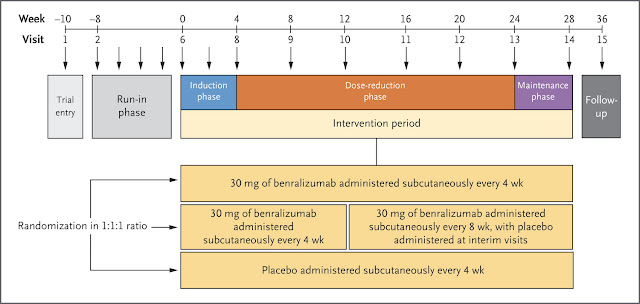
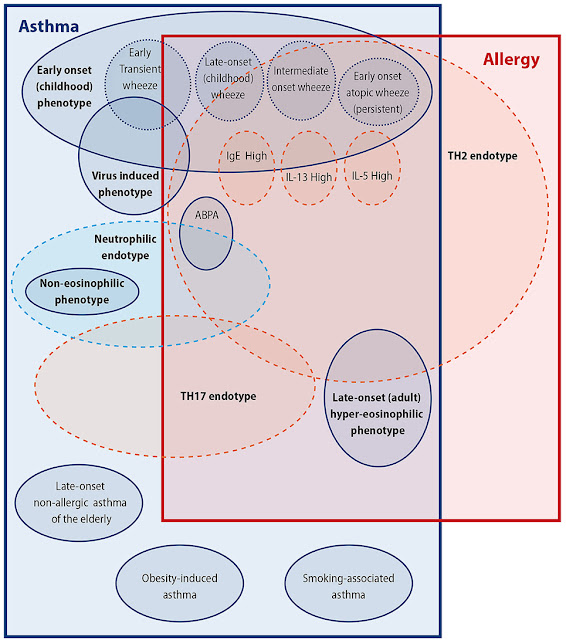
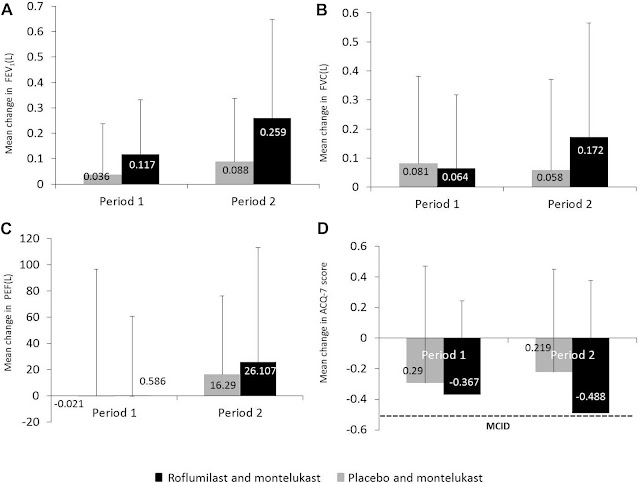
Today was published an important update to British Thoracic Society guidance on the management of asthma!
Diagnosing asthma
The guideline,
produced jointly by the British Thoracic Society (BTS) and the Scottish
Intercollegiate Guidelines Network (SIGN) emphasises that there is
still no single test that can definitively diagnose asthma and an
individual’s asthma status can change over time.
It recommends that if a health professional suspects asthma, they
should undertake a ‘structured clinical assessment’ using a combination
of patient history, examination and tests to assess the probability of
asthma.
The history should include a review of the following:
- Symptoms of cough, breathlessness, wheeze and chest tightness that have varied over time
- Any history of recurrent attacks of symptoms
- Any wheeze previously recorded by a health professional
- A personal or family history of allergic conditions such as eczema and allergic rhinitis
- Objective evidence of variability over time in obstruction to a patient’s airflow (using the results of lung function tests)
- The absence of any pointers to an alternative diagnosis to asthma.
Quality assured spirometry is spotlighted as the key frontline
breathing test to be performed in most situations with adults and
children over 5 years of age. It is important that spirometry is
quality assured i.e. professionals are trained and experienced in
preparing and delivering the test as well as analysing the results.
If the test shows obstruction to the patient’s airflow which reverses
with treatment, this strongly supports a diagnosis of asthma.
But a normal spirometry result does not always exclude an asthma
diagnosis – especially if a patient has no symptoms at the time. It may
be necessary for healthcare professionals to repeat spirometry when a
patient has symptoms, and/or use different breathing tests - and observe
over time.
One, often secondary, breathing test that can be carried out,
involves measuring an individual’s fractional exhaled nitric oxide
(FeNO) - a gas found in slightly higher levels in people with asthma. An
increase suggests inflammation of the airways, and supports, but
doesn’t prove, a diagnosis of asthma.
The guideline helps health professionals to assign patients into 3
groups based on the probability they have asthma; either high,
intermediate or low. It then summarises the key treatment and
management actions to be taken for each group.
If the probability of asthma is high, health professionals should
start a carefully monitored trial of treatment. If patients respond
well, according to lung function tests or symptom questionnaires, this
will confirm the diagnosis. Health professionals should code their
records as ‘suspected asthma’ until a diagnosis is confirmed and should
make a clear record on what basis the diagnosis was confirmed. If the
probability is low, further tests or immediate referral to a lung
specialist may be appropriate.
Treating asthma
The updated guideline also includes new or revised content in the
following areas: asthma drug treatment (replacing the previous stepwise
approach), non-drug treatments, supported self-management, and the role
of telehealthcare.
Key highlights include:
- Short acting beta2 agonists - a group of drugs that can provide
quick relief of asthma symptoms - are the key ‘rescue therapy’ from
symptoms or asthma attacks and can form part of all treatment plans, but
should rarely be used on their own
- A key emphasis on medication to prevent future asthma attacks -
inhaled corticosteroids remain the most effective ‘preventer’ drug for
all adults and children
- Asthma inhalers should not be prescribed generically to avoid
patients being given an unfamiliar device that they may not know how to
use properly
- If a patient has poor control of their asthma, it is essential to
check whether they are using their current drug treatment correctly and
regularly, before stepping up treatment
- Weight loss initiatives – including dietary and exercise programmes –
can be offered for overweight or obese adults and children with asthma
and may improve their asthma control
- Each patient should be offered a written asthma action plan as it is key to the effective management of their asthma
- The use of new electronic technologies can help in the delivery of
asthma care, and evidence shows they can be at least as good as
traditional methods, although outcomes do vary
Approaches include; games to encourage children to take their
medication, remote consultations, automated treatment reminders, and
computerised decision-support systems for health professionals. The
guideline says they can be considered according to local need
- Women with asthma who are pregnant should be informed of the
importance of continuing their asthma medication during pregnancy for
the health of both mother and baby
Dr John White, British Thoracic Society member and Consultant
Respiratory Physician, York NHS Foundation Trust, who co-chaired the
group that delivered the updated BTS/SIGN Guideline, said:
‘Asthma is a complex disease and symptoms can vary over time. In
addition, evidence shows there’s still no single ‘magic bullet test’ for
asthma. This all means that diagnosis isn’t always easy.
This update should be really valuable as it gives healthcare
professionals an evidence-based but highly practical approach to
suspecting and confirming a diagnosis of asthma, as well as giving the
latest guidance on the most appropriate treatments and interventions to
combat the disease.
The guideline also reinforces previous messages that remain vital in
the battle against asthma. It is critical, for example, that everyone
with the condition is offered a written personalised action plan and
review, and that inhalers are only prescribed after patients have
received training in using them and demonstrate adequate technique.
We do hope that health, social care and education professionals can
work together with people with asthma in using these guidelines to
provide the best care possible.’
SIGN is part of Healthcare Improvement Scotland.
Sara Twaddle, Director of Evidence for Healthcare Improvement Scotland, said:
“Over 5 million people are currently being treated for asthma in the
UK and 1,468 people died from asthma attacks in the UK in 2015 – the
highest level for 10 years. It’s important that we diagnose and treat
people to the best of our ability, hence the reason we update this
guideline for clinicians every two years using the most up-to-date
evidence. This new updated guideline underlines that there is still no
single diagnostic test for asthma, and emphasises the importance of
preventive therapy. We urge clinicians across the UK to refer to this
guideline for diagnosis and treatment. By doing so, they will help to
improve the care that people with asthma receive.”
The BTS/SIGN asthma guideline is a ‘living guideline’ updated
biennially. Following a scoping exercise, key sections are selected for
updating based on availability of new evidence.
Free full text: